Sidebar
HMGB1 ELISA Kit
04-HMGB1-ELISA 982 $
High mobility group box protein 1 (HMGB1), which was originally known as amphoterin, mediates neurite outgrowth and binds receptors for advanced glycation end products (RAGE). HMGB1 has 219 residues in its primary amino acid sequence, and there is >98% sequence identity between the HMGB1 of rodents and that of humans. In most cells, HMGB1 is located in the nucleus. It is a chromatin-associated nuclear protein that plays an important role in transcription and DNA recombination. Recently, HMGB1 has been shown to play a critical role in various acute and chronic diseases such as inflammation-mediated diseases, infective diseases, HIV, sepsis, tumors, cardiovascular, n$logic diseases, and amyloid pathologies. According to PubMed database, 41 journal articles regarding HMGB1 have been published during 2006 (as of September 13, 2006).
The HMGB1 ELISA kits and/or anti HMGB1 Abs for ELISA for quantitative determination of only HMGB1, are not crossreacting to HMGB2 which is highly conserved (>80% amino acid identity).
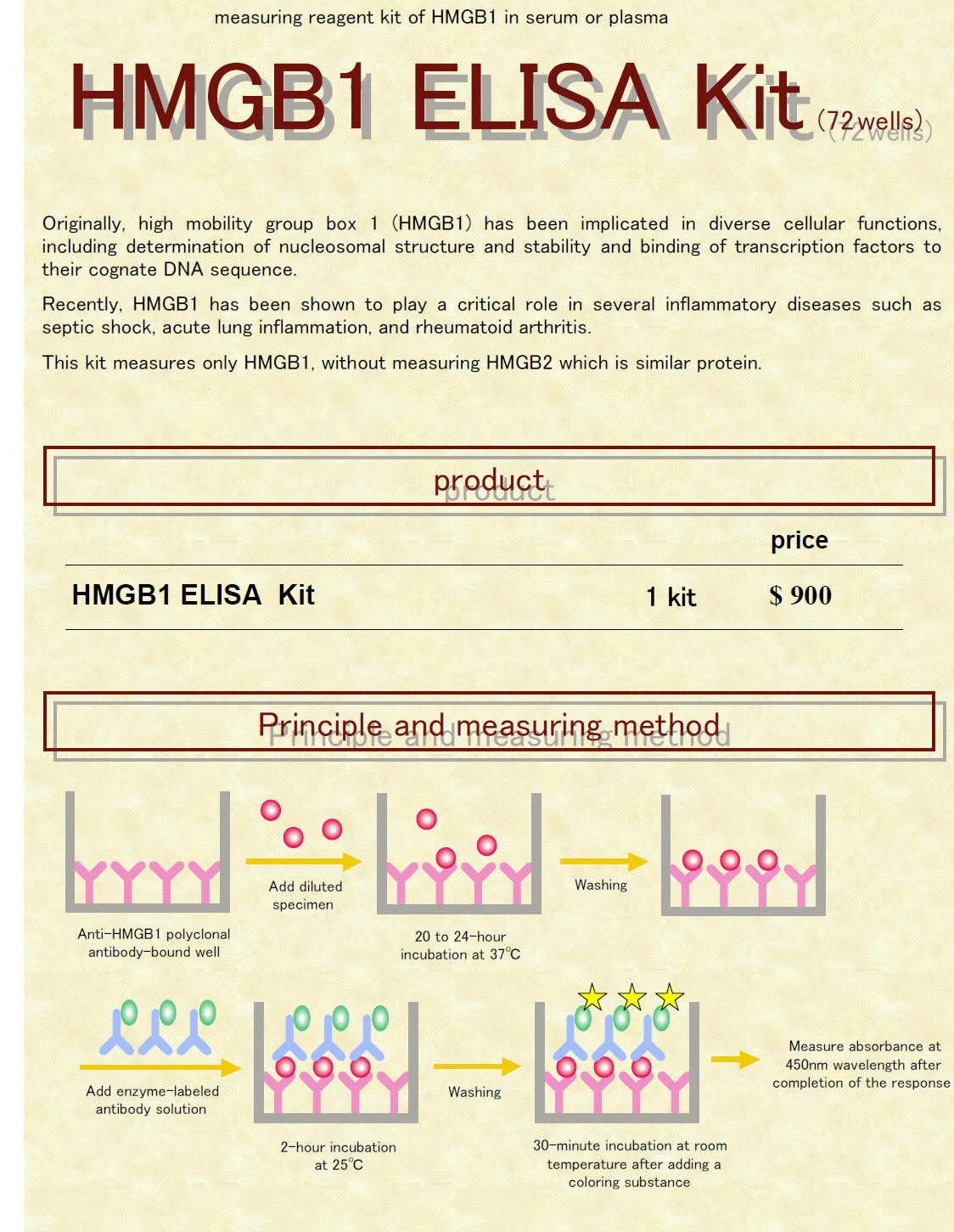
HMGB1 ELISA Kit The HMGB1 ELISA Kit is a 2-step sandwich ELISA.

HMGB1 can be determined using ELISA.
* ELISA Method: see [method] page.
* The HMGB1 ELISA kit contains all the reagents for measurement.
Please prepare a microplate washer and a microplate reader yourself.
HMGB1 can be specifically determined, so HMGB2 which has about 80%
homology in its amino acid sequence is not measured (less than 10%)
Serum and plasma of human, cattle, pigs, rabbits, rats, and mice can be measured using this kit. The results obtained show almost the same level.
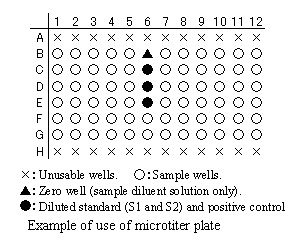
Measurement can be conducted using 72 wells of a 96well microplate.
 |
| 8-Wellx12 strips (antibody- coated) | 8 wells | 12 strips |
| Anti-HMGB1 polyclonal antibody | ||
| Standard (lyophilized) | 1 mL | 1 |
| Pig HMGB1 | ||
| Positive control (lyophilized) | 1 mL | 1 |
| Pig HMGB1 | ||
| Sample diluent solution | 20 mL | 1 |
| Buffer containing additives and preservative | ||
| Peroxidase-linked antibody(lyophilized) | 10 mL | 1 |
| Peroxidase-linked anti-HMGB1,2 monoclonal antibody | ||
|
Peroxidase-linked antibody dis solvent solution |
10 mL | 1 |
| Buffer containing additives and preservative | ||
| Substrate solution A | 5 mL | 1 |
| 3,3', 5,5'-Tetramethyl-benzidine, dihydrochloride, dihydrate | ||
| Substrate solution B | 5 mL | 1 |
| Buffer containing 0.005 mol/L hydrogen peroxide | ||
| Stop solution | 10 mL | 1 |
| 0.35 mol/L sulfuric acid | ||
| Wash solution (5x) | 100 mL | 2 |
| 5-Fold concentrated buffer containing Tween 20 | ||
| Plate seal | - | 2 |
Principle |
|
|
This figture is a reagent for HMGB1 quantitative determination using sandwich ELISA (enzyme-linked immunosorbent assay). A diluted specimen is added to the anti-HMGB1 polyclonal antibody-bound solid phase well, and HMGB1 in the specimen is specifically bound to the antibody. Next, an antigen-antibody complex (sandwich) is formed by adding peroxidase-labeled antibody. Color reaction is started by adding a coloring substance to the resulting complex, and absorbance is measured at 450nm wavelength after completion of the color reaction. |
|
 |
|
 |
|
<Method of Measurement> |
|
|
|
 |
|
|
||||||||||||||||
|
||||||||||||||||
| Literature | ||||||||||||||||
|
||||||||||||||||
| WHAT IS HMGB1? | ||||
| <Properties> | ||||
| 1. Is a protein whose molecular weight is about 30 kDa. | ||||
| 2. Is expressed in various cells. (Macrophages/ Monocytes, Endothelial cells, Neutrophils, Epithelial cells, Dendritic cells, Smooth muscle cells and so on) |
||||
| 3. Is involved in growth of dendrites of nerve cells. | ||||
| 4. Stabilizes with binding to DNA. | ||||
| 5. Serves as a transcriptional regulator. | ||||
| 6. Acts as a cytokine | ||||
|
||||
| <Scheme of domain structure> | ||||
|
||||
| <Functions of HMGB1 as a cytokine> | ||||
|
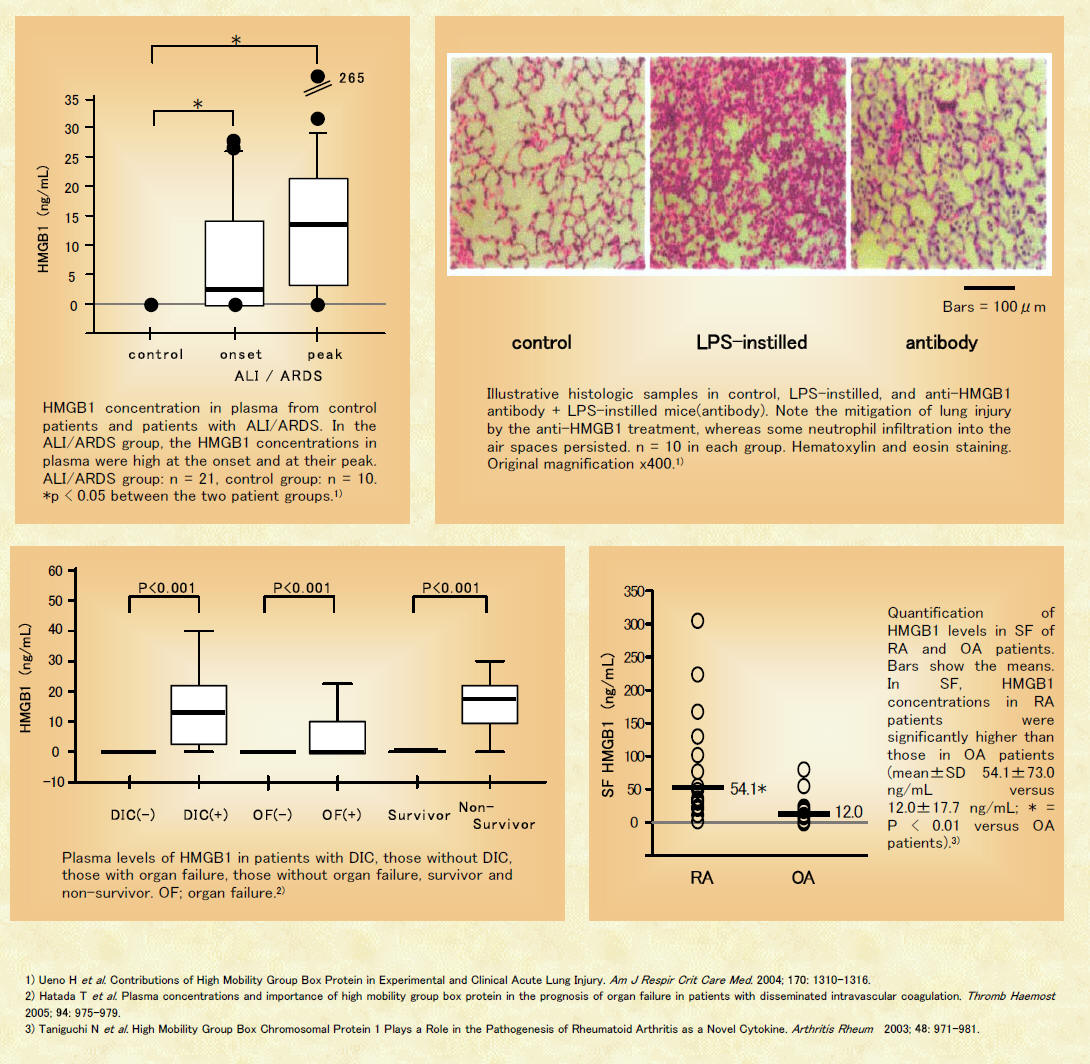
- References:
Am J Physiol Lung Cell Mol Physiol 288: L958-L965, 2005. First published January 7, 2005; doi:10.1152/ajplung.00359.2004
1040-0605/05 $8.00
HMGB1 contributes to the development of acute lung injury after hemorrhage
Jae Yeol Kim,1,5 Jong Sung Park,1 Derek Strassheim,1 Ivor Douglas,1 Fernando Diaz del Valle,1 Karim Asehnoune,1,6 Sanchayita Mitra,1 Sang Hyun Kwak,1,7 Shingo Yamada,2 Ikuro Maruyama,3 Akitoshi Ishizaka,4 and Edward Abraham1
1Division of Pulmonary Sciences and Critical Care Medicine, University of Colorado Health Sciences Center, Denver, Colorado 2Central Institute, Shino-Test Corporation, Sagamihara, Kanagawa, Japan 3Department of Laboratory and Molecular Medicine, Faculty of Medicine, Kagoshima University, Kagoshima, Japan 4Department of Medicine, Keio University, School of Medicine, Tokyo, Japan 5Department of Internal Medicine, Chung Ang University College of Medicine, Seoul, Korea 6Service d'Anesthesie-Réanimation et Unité Propre de Recherche de l'Enseignment Superieur-Equipe d'Accueil, Hospital de Bicêtre, Le Kremlin Bicetre, France 7Department of Anesthesiology, Chonnam University Medical School, Gwangju, Korea
Submitted 21 September 2004 ; accepted in final form 3 January 2005
| ABSTRACT |
|---|
High mobility group box 1 (HMGB1) is a novel late mediator of inflammatory responses that contributes to endotoxin-induced acute lung injury and sepsis-associated lethality. Although acute lung injury is a frequent complication of severe blood loss, the contribution of HMGB1 to organ system dysfunction in this setting has not been investigated. In this study, HMGB1 was detected in pulmonary endothelial cells and macrophages under baseline conditions. After hemorrhage, in addition to positively staining endothelial cells and macrophages, neutrophils expressing HMGB1 were present in the lungs. HMGB1 expression in the lung was found to be increased within 4 h of hemorrhage and then remained elevated for more than 72 h after blood loss. Neutrophils appeared to contribute to the increase in posthemorrhage pulmonary HMGB1 expression since no change in lung HMGB1 levels was found after hemorrhage in mice made neutropenic with cyclophosphamide. Plasma concentrations of HMGB1 also increased after hemorrhage. Blockade of HMGB1 by administration of anti-HMGB1 antibodies prevented hemorrhage-induced increases in nuclear translocation of NF-![]() B in the lungs and pulmonary levels of proinflammatory cytokines, including keratinocyte-derived chemokine, IL-6, and IL-1
B in the lungs and pulmonary levels of proinflammatory cytokines, including keratinocyte-derived chemokine, IL-6, and IL-1![]() . Similarly, both the accumulation of neutrophils in the lung as well as enhanced lung permeability were reduced when anti-HMGB1 antibodies were injected after hemorrhage. These results demonstrate that hemorrhage results in increased HMGB1 expression in the lungs, primarily through neutrophil sources, and that HMGB1 participates in hemorrhage-induced acute lung injury.
. Similarly, both the accumulation of neutrophils in the lung as well as enhanced lung permeability were reduced when anti-HMGB1 antibodies were injected after hemorrhage. These results demonstrate that hemorrhage results in increased HMGB1 expression in the lungs, primarily through neutrophil sources, and that HMGB1 participates in hemorrhage-induced acute lung injury.
high mobility group box 1; nuclear factor-![]() B; neutrophils
B; neutrophils
ACUTE LUNG INJURY (ALI) is frequently associated with trauma and blood loss (2–4, 6, 8, 10, 12, 14, 15, 19, 23, 26, 27, 29). ALI is characterized by an intense inflammatory process in the lungs, with accumulation of activated neutrophils and the development of interstitial edema (3, 4,25). Activation of the transcriptional regulatory factor NF-B is also increased in ALI, providing an explanation for the increases in proinflammatory cytokines, such as TNF- and IL-1, whose expression is modulated by this molecule (19, 27). High mobility group box 1 (HMGB1) protein, originally identified as a DNA binding protein, also has potent proinflammatory properties. Exposure of neutrophils or macrophages to HMGB1 induces nuclear translocation of NF-B and enhanced production of proinflammatorycytokines, including TNF- and IL-1, at least in part through interaction of HMGB1 with Toll-like receptor (TLR)-2, TLR-4, and the receptor for advanced glycation end products (10, 21, 22). In murine experiments, serum concentrations of HMGB1 increase 8–32 h after administration of LPS or TNF- (32). Systemic administration of purified recombinant HMGB1 is lethal in mice (5). Intratracheal injection of HMGB1 results in the development of acute pulmonary inflammation, and blockade of HMGB1 decreases the severity of LPS-induced ALI, implicating HMGB1 as a mediator of sepsis-associated lung injury (1). Specific inhibition of HMGB1 activity with anti-HMGB1 antibodies beginning as late as 24 h after endotoxemia or the induction of bacterial peritonitis increased survival (34).
In a report from a single patient suffering from life-threatening blood loss, circulating levels of HMGB1 were found to be elevated (20). However, there was no evidence of organ dysfunction in that patient, and the contribution of HMGB1 to ALI, organ system abnormalities, or mortality after hemorrhage have not been investigated. In the present experiments, we found that HMGB1 levels increase in the lung and plasma after blood loss and that administration of anti-HMGB1 antibodies, even after hemorrhage, can ameliorate the severity of ALI. Such results demonstrate that HMGB1 contributes to hemorrhage-induced ALI.
| MATERIALS AND METHODS |
|---|
Mice. Male BALB/c mice, 8–12 wk of age, were purchased from Harlan Sprague Dawley (Indianapolis, IN). The mice were kept on a 12-h light/dark cycle with free access to food and water. All experiments were conducted in accordance with protocols approved by the University of Colorado Health Sciences Center Institutional Animal Care and Use Committee. Chemicals and reagents. Polyclonal rabbit anti-HMGB1, polyclonal chicken IgY anti-HMGB1, and control chicken IgY antibodies were gifts from Shino-Test (Sagamihara, Kanagawa, Japan) (33). Polyclonal rabbit anti-mouse -actin antibody was obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Isoflurane was obtained from Abbott Laboratories (Chicago, IL). Evans blue dye (EBD), N-ethylmaleimide, o-DA (3,3'-dimethoxybenzidine dihydrochloride), hexadecyltrimethylammonium bromide, and all other chemicals were obtained from Sigma (St. Louis, MO).
Model for hemorrhage. The mouse hemorrhage model used in these experiments has been described previously (3). In brief, mice were anesthetized with inhaled isoflurane. Cardiac puncture was used to remove 30% of the calculated total blood volume (0.27 ml/10 g body wt) over 60 s into a heparanized syringe. One hour after the induction of hemorrhage, mice were again anesthetized with isoflurane, and the previously removed blood was infused into the retroorbital venous plexus. We previously demonstrated that heparin itself does not contribute to the development of ALI after hemorrhage (2). The sham procedure involved cardiac puncture under isoflurane anesthesia, without blood removal, followed by a second episode of anesthesia and retroorbital puncture 1 h later. In previous studies (26), we found that mean arterial blood pressure fell to 40 mmHg immediately after blood withdrawal, with return to baseline, prehemorrhage levels at 1 h after hemorrhage, the time that anti-HMGB1 and control antibodies were administered.
Generation of neutropenia. Neutropenia was induced using cyclophosphamide, as previously described by our laboratory (3). In brief, mice were given 150 mg/kg of cyclophosphamide intraperitoneally (ip) in 0.2 ml of PBS 1 and 4 days before hemorrhage. Control mice were given0.2 ml of PBS ip at the same time points. The effects of cyclophosphamide treatment on neutrophil numbers were determined by preparing Wright's stains on peripheral blood smear. In mice treated with this cyclophosphamide regimen, there was >99% reduction in peripheral blood neutrophil numbers compared with those present in control mice.
Administration of anti-HMGB1 antibody. Either neutralizing polyclonal chicken IgY anti-HMGB1 antibody (200 µg/mouse) or control chicken IgY antibody (200 µg/mice) were injected into the peritoneum 1 h after the induction of hemorrhage (immediately after the reinfusion of the aspirated blood). The therapeutic effects of both antibodies were evaluated at 4 h (for cytokines, MPO activity, and NF-B activity in the lung) and at 24 h (for lung leak by EBD assay) after the induction of hemorrhage.
Preparation of lung homogenate for ELISA and Western blot analysis. Lung tissues were homogenized as previously described (3). In brief, lung samples were homogenized in ice cold lysis buffer (50 mM HEPES, 150 mM NaCl, 10% glycerol, 1% Triton X-100, 1.5 mM MgCl2, 1 mM EGTA, 1 mM sodium vanadate, 10 mM sodium pyrophosphate, 10 mM NaF, 300 µM p-nitrophenyl phosphate, 1 mM PMSF, 10 µg/ml leupeptin, and 10 µg/ml aprotinin, pH 7.3) containing 1 mM protease inhibitor (Sigma). Homogenates were centrifuged at 14,000 g for 15 min, and supernatants were collected. The protein concentration of each sample was assayed using the micro-BCA protein assay kit standardized to BSA, according to manufacturer's protocol (Pierce, Rockford, IL).
Western blot analysis. Western blotting was used to determine HMGB1 levels in the lungs. Briefly, 100 µg of lung homogenate protein were loaded on a 10% Tris·HCl-SDS-polyacrylamide gel and run for 1 h at 120 V. Protein was electrotransferred to a nitrocellulose membrane and then blocked with 5% nonfat dry milk and Tris-buffered saline with 0.1% Tween 20. After being blocked, the membrane was incubated overnight at 4°C with a specific polyclonal rabbit primary antibody to HMGB1 (Shino-Test) at a dilution of 1:2,000 followed by anti-rabbit horseradish peroxidase-coupled secondary antibody (Bio-Rad, Hercules, CA) at a dilution of 1:5,000. After three washings, bands were detected using enhanced chemiluminescence plus Western blotting detection reagents (Amersham Pharmacia Biotech, Piscataway, NJ). The membranes were then stripped using stripping buffer (63 mM Tris·HCl, pH 6.8, 2% SDS, and 100 mM 2-mercaptoethanol; Bio-Rad) and reprobed with antibodies specific for -actin (Santa Cruz Biotechnology) to ensure equal loading of protein on the gel.
Immunohistochemical analysis. For each antigen, lung tissues from control and hemorrhaged mice were stained simultaneously. After paraffin-embedded blocks had been cut into 5-µm sections and mounted onto slides, the specimens were deparaffinized and rehydrated. High-temperature antigen retrieval involved boiling the slides in citrate buffer (10 mM per liter, pH 6.0) for 20 min, followed by incubation with the avidin-biotin-peroxidase complex (Dako Ark Animal Research Kit, peroxidase) according to the manufacturer's instructions. For the detection of HMGB1, the samples were incubated with primary antibody (polyclonal chicken IgY anti-HMGB1 antibody, dilution 1:200) at room temperature for 15 min. The samples were then stained with O-DA as the chromogen and counterstained with Mayer's hematoxylin. Normal blocking serum without primary antibody was used for the negative control.
Cytokine and HMGB1 ELISA. Immunoreactive TNF-, IL-1, macrophage inflammatory protein-2 (MIP-2), keratinocyte-derived chemokine (KC), IL-6, and IL-10 were quantitated in duplication using commercially available ELISA kits (R&D Systems, Minneapolis, MN) according to the manufacturer's instructions and as described previously (36). ELISA for HMGB1 in the plasma was performed with the use of monoclonal antibodies to HMGB1 and with standardization to a curve of recombinant human HMGB1 (33).
MPO assay. MPO activity was assayed as reported previously with minor modifications (23). In brief, lung tissue was homogenized in 1 ml of 50 mM potassium phosphate buffer (pH 6.0) containing the reducing agent N-ethylmaleimide (10 mM) for 30 s on ice. The homogenate was centrifuged at 12,000 g for 30 min at 4°C. The proteinous pellet was homogenized once more in ice-cold buffer, and the homogenate was centrifuged. The pellet was resuspended and sonicated on ice for 90 s in 10x volume of hexadecyltrimethylammonium bromide buffer (0.5% in 50 mM potassium phosphate, pH 6.0). Samples were incubated in a water bath (56°C) for 2 h and then centrifuged at 12,000 g for 10 min. The supernatant was collected for assay of MPO activity as determined by measuring the H2O2-dependent oxidation of o-DA at 460 nm.
Assessment of lung leak. EBD was used to assess lung leak. EBD solution (5 mg/ml, 200 µl) was injected through a tail vein (50 mg/kg). One hour later, animals were anesthetized with inhaled isoflurane, and the chest was opened. The pulmonary vasculature was flushed free of blood by gentle infusion of 10 ml of PBS into the beating right ventricle. The lungs were then excised, weighed, and dried at 60°C for 24 h and then placed in 3 ml of formamide at 60°C for 36 h to extract EBD. Dye content was evaluated by spectrophotometry at 620 nm (16).
Preparation of nuclear extracts from whole lung samples. Nuclear extracts were prepared from the lung tissue as previously described with minor modification (3). In brief, lungs were snap-frozen in liquid nitrogen and then homogenized in buffer A (10 mM HEPES, pH 7.9, 1.5 mM MgCl2, 10 mM KCl) containing 1 mM DTT and 1 mM protease inhibitor cocktail for use with mammalian cell and tissue extracts that contain 4-(2-aminoethyl)benzenesulfonyl fluoride, pepstatin A, E-64, bestatin, leupeptin, and aprotinin (Sigma). After homogenates were stored on ice for 15 min, 10% Igepal CA630 solution (nonionic surfactant used as an emulsifier) was added to a final concentration of 0.6%. Homogenates were then centrifuged immediately at 4°C for 1 min at 8,000 g. After the supernatant was removed, the nuclear pellet was resuspended in 75 µl of extraction buffer C [20 mM HEPES, pH 7.9, 1.5 mM MgCl2, 0.42 M NaCl, 0.2 mM EDTA, 25% (vol/vol) glycerol] containing 1 mM DTT and 1 mM protease inhibitor. The extract was centrifuged at 4°C for 15 min at 18,000 g. The supernatant was collected and stored at –80°C.
Electrophoretic mobility shift assay. Nuclear extracts (20 µg) were incubated at room temperature for 15 min in 20 µl of reaction buffer containing 10 mM Tris·HCl (pH 7.5), 1 mM MgCl2, 0.5 mM EDTA, 0.5 mM DTT, 50 mM NaCl, and 4% glycerol with 32P-end labeled, double-stranded oligonucleotide probe specific for the B site 5'-GCCATGGGGGGATCCCCGAAGTCC-3' (Active Motive, Carlsbad, CA) and 1 µg of poly(dI-dC)·poly(dI-dC). The complexes were resolved on 5% polyacrylamide gels in Tris·HCl (pH 8.0)-borate-EDTA buffer at 10 V/cm. Dried gels were exposed with Kodak Biomax MS film (Rochester, NY) for 1–24 h at –70°C. Quantification was performed by image analysis using densitometry (ChemiDoc system, Bio-Rad) (22).
Statistical analysis. Data are expressed as means ± SE. ANOVA was performed with SPSS Windows 9.0 statistical analysis software, and a difference was accepted as significant if the P value was <0.05, as verified by Duncan's and Tukey's post hoc test.
| DISCUSSION |
|---|
HMGB1 has been demonstrated to be an important mediator of mortality and organ system dysfunction, including ALI, in models of infection, such as bacterial peritonitis and endotoxin exposure (1, 5, 13, 30, 32, 35). In those settings, blockade of HMGB1 with specific antibodies or the HMGB1 A box fragment improves survival and diminishes circulating levels of proinflammatory cytokines (34). In addition, HMGB1 itself can produce organ injury, as shown by the development of ALI after its intratracheal administration (1). However, a role for HMGB1 in contributing to the development of ALI and other organ dysfunction in pathophysiological settings, such as severe hemorrhage, that are not associated with exposure to bacteria or bacterial products has not been previously characterized.
Endotoxemia is not routinely observed in severely injured trauma patients suffering from severe blood loss or in murine models of hemorrhage, indicating that mediators other than endotoxin are likely to be responsible for initiating the inflammatory response that leads to ALI and other organ dysfunction in these settings (8, 15, 26). Tissue ischemia, with release of reactive oxygen intermediates (ROI), occurs after blood loss (17, 27, 29). We and others have shown that ROI contribute to hemorrhage-induced activation of NF-B in the lungs as well as expression of NF-B-dependent proinflammatory cytokines and the development of ALI (11, 12, 27). ALI is characterized by the accumulation in the lungs of activated neutrophils that demonstrate increased nuclear concentrations of NF-B and produce elevated amounts of proinflammatory cytokines. These pulmonary neutrophils play a major role in the development of hemorrhage or endotoxemia-induced lung injury (3, 23, 27). Exposure of neutrophils to ROI, such as H2O2, recapitulates many of the characteristics that are found among pulmonary neutrophils in ALI, such as NF-B activation and production of the cytokine TNF- (7, 28). Such ROI-induced events that result in enhanced neutrophil proinflammatory properties appear to be at least partially a result of activating the kinases phosphatidylinositol 3-kinase (PI3K) and Akt (18, 31). Of note, signaling pathways involving PI3K have been shown to be important in the HMGB1-induced neutrophil activation (21).
In the present study, pulmonary levels of HMGB1 were increased as soon as 4 h after hemorrhage and remained elevated for >72 h (Fig. 1, A and B). Neutrophils appeared to be the primary contributors to posthemorrhage increases in pulmonary HMGB1 levels, since no elevations in lung concentrations of HMGB1 were found in mice rendered neutropenic by cyclophosphamide treatment. The role of neutrophils in contributing to posthemorrhage increases in pulmonary HMGB1 levels is supported by the fact that lungs of hemorrhaged mice were infiltrated with neutrophils that were strongly positive for HMGB1. The present findings, showing an important role for neutrophils as contributors to the pulmonary inflammatory response accompanying ALI, are consistent with our previous studies (3) showing that the severity of hemorrhage-inducedlung injury is decreased after elimination of neutrophils.
Concentrations of proinflammatory mediators other than HMGB1, such as IL-1 and KC, were found only to be elevated in the lungs 4 h after hemorrhage, but not at later time points (Fig. 3). Such results, showing persistent elevations in HMGB1 after other proinflammatory cytokines were no longer detectable, have been reported in models of endotoxemia and sepsis and point to a role for HMGB1 as a late-acting mediator of inflammation and organ dysfunction (32, 35). Consistent with lung injury being a response to proinflammatory mediators with a delayed appearance, increases in lung leak, as measured by EBD extravasation, were only found 24 h after hemorrhage, a time point when both pulmonary and circulating levels of HMGB1 were elevated (Fig. 4B).
Blockade of HMGB1 with anti-HMGB1 antibodies diminished hemorrhage-induced increases in pulmonary levels of inflammatory cytokines, nuclear translocation of NF-B, and neutrophil accumulation and the development of interstitial edema in the lungs. Such findings demonstratethat HMGB1 contributes to the development of ALI after severe blood loss. These experiments, although showing an important role for HMGB1 in hemorrhage-induced lung injury, also suggest that other mediators are involved in this pathophysiological process. In particular, pulmonary levels of IL-1 and KC, although diminished in anti-HMGB1-treated mice, still remained significantly above baseline levels. IL-1 is released by multiple pulmonary cell populations, including neutrophils, macrophages, and endothelial and epithelial cells, and has been shown to contribute to hemorrhage-induced ALI (3, 9, 14, 27). Given the persistent elevations in IL-1 and KC, a C-X-C chemokine with potent neutrophil chemoattractant properties, it is not surprising the neutrophils continue to accumulate in the lungs of the anti-HMGB1-treated mice, as shown by significant elevations in MPO levels. Interestingly, though, the amount of lung leak in animals treated with anti-HMGB1 antibodies after hemorrhage is not different from that found in control, unmanipulated mice. Such results indicate that HMGB1 may contribute to the development of tissue injury through pathways that are not solely dependent on inflammatory processes. Of note, HMGB1 has been shown to directly increase the permeability of enterocyte monolayers and impair intestinal barrier function through a mechanism that depends on the formation of nitric oxide and peroxynitrite (24). Similar effects on epithelial tight junctions in the lungs by HMGB1 would lead to the development of interstitial pulmonary edema, independent of any proinflammatory actions.
In the present experiments, anti-HMGB1 antibodies were administered 1 h after hemorrhage and were still able to ameliorate the severity of ALI. Such results may have important therapeutic implications. Trauma associated with blood loss often occurs in otherwise healthy victims. With rapid response prehospital services, such patients are usually seen by paramedics and are in the hospital within minutes of injury. At these early time points, ALI has not yet developed, and therapies such as anti-HMGB1 antibodies may be of use in preventing ALI and other organ dysfunctions associated with severe blood loss.